Aseptic - “an environment or procedure that is free of contamination by pathogens”
Bauman, Robert W. Microbiology San Francisco: Pearson Education Inc., 2004.
Introduction
In the microbiology lab we use aseptic technique to:
- Prevent contamination of the specific microorganism we are working with.
- Prevent contamination of the room and personnel with the microorganism we are working with.
Many of the microorganisms we will be working with in lab are known pathogens. Proper and appropriate aseptic technique is vitally important for the safety of all lab personnel; it is also essential for the successful completion of the lab portion of this class. The skills and awareness you develop practicing aseptic technique will carry over to your career as a health professional. Many of our former students comment that this is the most important thing they learned in lab!
In this lab you will be learning standard microbiological procedures appropriate for Biosafety Level (BSL) 1 and Biosafety Level (BSL) 2 precautions. We follow the safety guidelines established by the Center for Disease Control and Prevention (CDC). Complete documentation is available at the CDC website.
- BSL1 – “suitable for work involving well-characterized agents not known to consistently cause disease in healthy adult humans, and of minimal potential hazard to laboratory personnel and the environment”.
- BSL2 – “suitable for work involving agents of moderate potential hazard to personnel and the environment”.
- Overview
- Part I - Procedures for Practice "Organisms"
- Materials
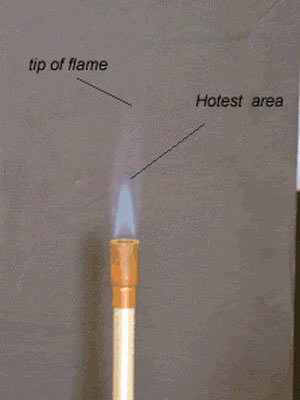
- Using a Bunsen Burner
- Broth to Broth Transfers Using BSL2 Procedures
- Broth to Plate Transfers Using BSL2 Procedures
- Part II - Procedures for BSL2 Organisms
- Materials
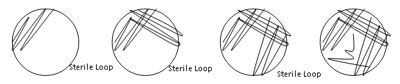
- Plate to Plate Transfers Using BSL2 Procedures
- Plate to Broth Transfers Using BSL2 Procedures
- Disposal Instructions