The information presented in this lab is from The Manual of Clinical Microbiology, 8th Ed. The procedures are paraphrased from the National Committee for Clinical Laboratory Standards (NCCLS) 2000. Approved Standard. M2-A7.
Introduction
It is not enough to just identify your organism. You also need to know what antimicrobial agents your organism is susceptible to. There are several methods to determine this.
Dilution testing is used to quantitatively determine the minimal concentration (in mg/ml) of antimicrobial agent to inhibit or kill the bacteria. This is done by adding two-fold dilutions of the antimicrobial agent directly to an agar pour, a broth tube, or a micro-broth panel. The lowest level that inhibits the visible growth of the organism is considered the Minimum Inhibitory Concentration (MIC). The agar pour method is considered the reference test procedure in Europe. The broth dilution method is more widely accepted in North America. The E test (AB Biodisk) is a plastic strip with a gradient concentration of antimicrobial agents impregnated in it. The strip is placed directly on the surface of an inoculated plate. The MIC is read from the strip where the growth inhibition intercepts the disk. These strips are relatively expensive.
Many physicians however do not need no know the exact MIC, but just which antibiotics the pathogen is susceptible, intermediate, or resistant to. The Kirby-Bauer agar diffusion method is well documented and is the standardized method for determining antimicrobial susceptibility. White filter paper disks (6 mm in diameter) are impregnated with known amounts of antimicrobial agents. Each disk is coded with the name and concentration of the agent. For example, 10 µg of Ampicillin is indicated on the disk by AM-10. The code is listed on the Disk Zone Diffusion Diameter Chart.
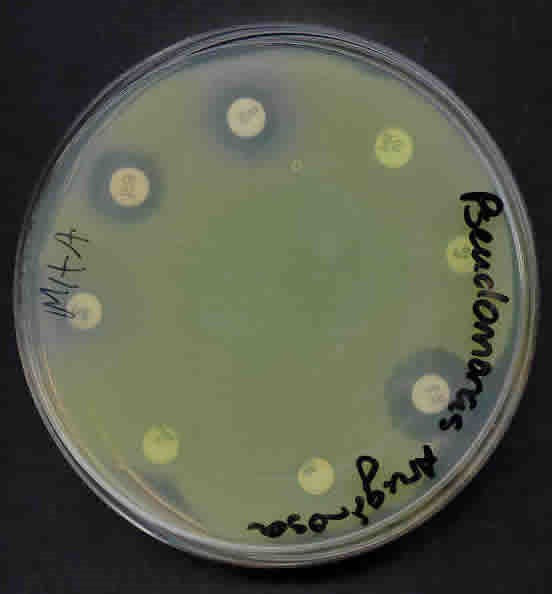
Pseudomonas aeruginosa on MHA incubated at 37°C for 24 hours.
The impregnated disks are placed on an inoculated Mueller Hinton Agar (MHA) plate. The drug diffuses through the agar. The plates are incubated for 16-24 hours. The agar may be supplemented with blood or you may use blood agar for fastidious organisms. The diameter of the visible zone of inhibition is measured and compared to reference values. There should be sufficient bacteria to form a visible lawn of growth where it is not inhibited by the drug.
The results are interpreted qualitatively as resistant, intermediate, or susceptible. The standard protocol must be followed exactly for you, or any clinical lab, to interpret the results reliably.
There may be some inhibition of growth and the organism could still be considered resistant to that antimicrobial agent if the zone diameter is smaller than the reference values listed on the chart. Also note that different antimicrobial agents have different measurements for resistant, intermediate, and susceptible.
A zone of inhibition may be considered susceptible for one antimicrobial agent and not for another. For example, in order for ampicillin (AM-10) to be an effective antimicrobial agent, the zone of inhibition for enterics and most streps must be greater than 16 mm while for staphs it must be greater than 28 mm.
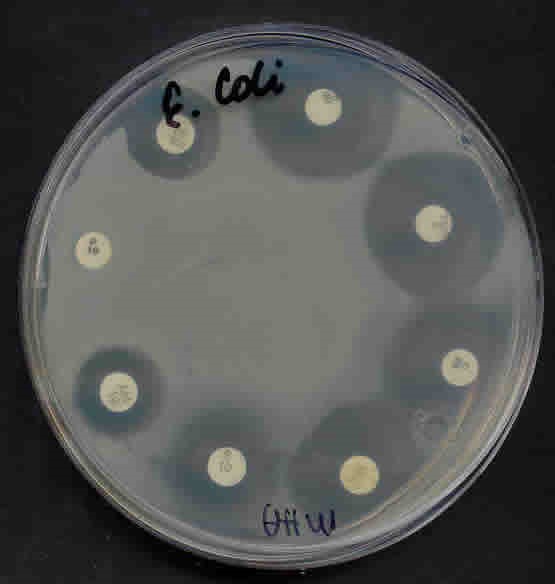
E. coli on MHA incubated at 37°C for 24 hours.
When determining which antimicrobial agents are best for treatment when multiple zones of inhibition are present, be sure to look at the relative zone of inhibition for that particular antimicrobial agent and compare your measurements to that.
For example, let’s say that your enteric organism has a zone of inhibition around the Polymixin B disk of 20 mm and a zone of inhibition around the Tetracycline disk of 20 mm. Because these measurements are larger than the susceptibility zones listed on the Disk Zone Diffusion Diameter Chart, both of these antibiotics would be considered as possibilities for treatment.
However, when we look more closely, we see that a 20 mm zone of inhibition for Tetracycline is only 1 mm larger than what is required to be susceptible while a 20mm zone of inhibition for Polymixin B is 8mm larger than the minimum susceptibility measurement needed. In this particular case then, Polymixin B and Tetracycline would both be adequate for treatment, but the Polymixin B would be the best choice.